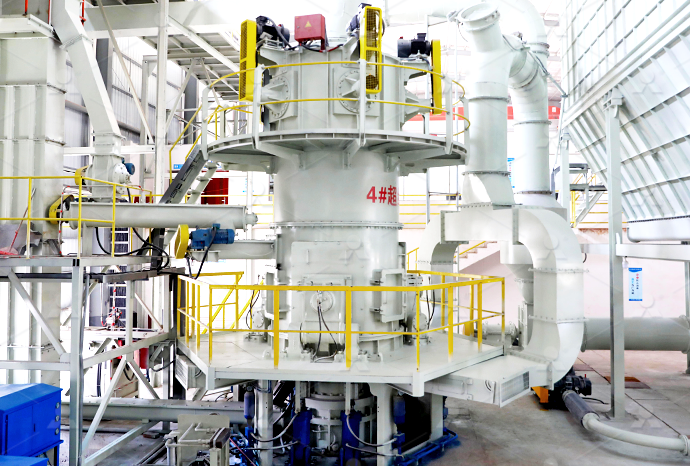
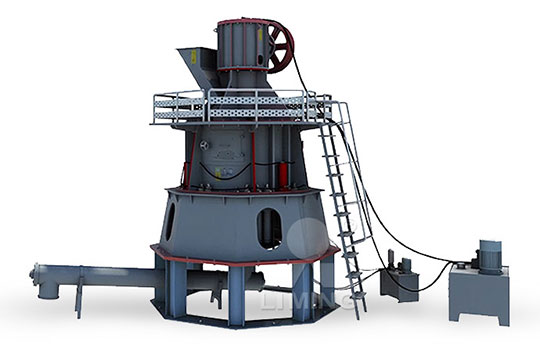
How Much is Quicklime Calcium Oxide Limestone Deep Processing Equipment

Calcium Oxide (quicklime) Cales de Llierca
Our calcined products are produced in energy efficient regenerative kilns where Calcium Oxide (quicklime) is obtained The later grinding and screening process generates the different calcium oxide products from granular products to highly reactive micronized limeCalcium oxide within quicklime reacts readily with water, liberating 267 kcal/kg CaO, as follows: CaO + H2O –> Ca(OH) + heat The reaction takes place at an average temperature of 100 ̊C Developing a modular lime plant CimprogettiQuicklime is produced by heating crushed and sorted limestone in a rotary or shaft kiln The limestone (CaCO 3) is broken down into calcium oxide (CaO), ie, quicklime, and carbon Quicklime NordkalkQuicklime, also referred to as lime (calcium oxide (CaO)), is derived from high quality, natural deposits of limestone (calcium carbonate (CaCO 3)) or dolomitic limestone (calcium Quicklime (Calcium Oxide) Mintek Resources

High Calcium Quicklime Graymont
High calcium quicklime, or calcium oxide (CaO) is a white alkaline, crystalline solid widely used across many essential applications The production of high calcium quicklime begins with the 1 Quarrying of raw limestone, crushing and sizing 2 Calcining (burning) limestone in a kiln or calciner 3 Processing the lime further by hydrating, mixing with water if necessary (Not all CO2 Mitigation CalixQuick Lime is the result of heating the high purity Limestone (9799% CaCO3), after being crushed to suitable particle size in vertical Kilns and applying a high temperature running Quicklime – Saudi LimeQuicklime is manufactured by chemically transforming calcium carbonate (limestone – CaCO3) into calcium oxide Hydrated lime is created when quicklime chemically reacts with water It is LIMETREATED SOIL CONSTRUCTION MANUAL Graymont

Calcium oxide Wikipedia
Its usage varies from about 30 to 50 kilograms (65–110 lb) per ton of steel The quicklime neutralizes the acidic oxides, SiO 2, Al 2 O 3, and Fe 2 O 3, to produce a basic molten slag 2021年12月20日 The Importance of Calcium Carbonate Calcium carbonate (CaCO3) comprises more than 4% of the earth’s crust and is found worldwide Its most common natural forms are chalk, limestone, and marble (produced by Calcium Carbonate Manufacturing Process and High calcium quicklime (CaO) is produced when limestone, or calcium carbonate (CaCO 3), is heated in a kiln through the process of calcination CaCo 3 + heat > Cao = CO 2 After limestone with high calcium content is sourced from our High Calcium Quicklime Carmeuse2023年4月24日 This process, known as calcination, causes the limestone to decompose into calcium oxide and carbon dioxide Quicklime processing The calcium oxide produced in the lime kiln is then processed further to remove How is calcium oxide manufactured industrially?
.jpg)
Quick Lime Preparation, Properties and Uses Hebei Yayang
2023年10月11日 Quicklime (Calcium Oxide) quicklime is one of many reagents offered by Mintek Resources Quicklime, also referred to as lime (calcium oxide (CaO)), is derived from high quality, natural deposits of limestone (calcium carbonate (CaCO3)) or dolomitic limestone (calcium magnesium carbonate (CaCO3 + MgCO3) Quicklime is produced by heating the The conversion of limestone into quicklime (calcium oxide) and slaked lime (calcium hydroxide Chemguide: Core Chemistry 14 16 Just for the record, an igneous rock is one formed by the welling up of molten material from very deep in the earth limestone, quicklime and slaked lime chemguideQuicklime Supplier, Limestone, Calcium Oxide Manufacturers/ Suppliers Shandong CITIC Calcium Industry Co, Ltd The average calcium oxide content is 54% min with stable quality we shall continue researching and developing new products in the deep processing field of limestone, such as food grade hydrated lime, stonepaper making, Quicklime Manufacturer, Limestone, Calcium Oxide Supplierchemical reaction, so calcium hydroxide contains 757% CaO and 243% H 2O The process of adding water to calcium oxide to produce calcium hydroxide is referred to as hydration process or lime slaking The hydration of CaO, commercially referred to as quick lime, is an exothermic process releasing a great quantity of heatAn Overview of Lime Slaking and Factors That Affect the Process

What Is Quicklime And How Does It Work? Medium
2023年2月10日 Quicklime, also known as calcium oxide (CaO), is a white or gray powder that is created by heating limestone to a high temperature, typically above 900°C (1652°F) This process, known as 2024年2月27日 Quicklime is produced through the thermal processing of limestone in industrial kilns During quarry operations, fine particulate quarry dust adheres to limestone lump surfaces, increasing the bulk concentration of impurities in limestone products During thermal processing in a kiln, impurities such as Si, Mg, Al, Fe, and Mn react with Ca, reducing quicklime product Impact of Limestone Surface Impurities on Quicklime Product 2015年5月4日 Ignite the residue in a platinum crucible, blast, cool in a desiccators and weigh as aluminium oxide and ferric oxide Make up the filtrate to 250 ml Pipette out 50 ml of the filtrate in a beaker and dilute to 100 ml Heat to boiling and add slowly about 35 ml of boiling ammonium oxalate solutionDETERMINATION OF TOTAL CALCIUM OXIDE IN LIME (IS: 1514 and washing it removes impurities Calcining is the heating of limestone to convert the calcium carbonate into calcium oxide This process is typically carried out in a rotary or vertical shaft kiln Required temperatures of the kilns exceed 1800 degrees The product of calcining is quicklime which can be used as "pebble lime" or may be crushed orLimestone and Crushed Rock Department of Energy

Type Selection of Quicklime Deep Processing Equipment
2022年12月22日 Quicklime, the main component of which is calcium oxide, is calcined from limestone and is generally used in steel mills, stainless steel plants, furnace charge plants, etc The main processing fineness is less than 3mm, 100 Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO 3; mineral calcite) in a lime kiln This is accomplished by heating the material to above 825 °C Calcium oxide Wikipedia2024年4月15日 Lime, primarily composed of calcium carbonate (CaCO3), is used in cooking and agriculture, while quicklime, or calcium oxide (CaO), is a caustic alkaline substance used in cement and environmental applicationsLime vs Quicklime — What’s the Difference?l Limestone quality l Calcium oxide properties (size and reactivity) l Chemical properties and water quantity l Retention time for evaluating the kinetic reaction l Mixing velocity l Steam formation and final moisture Calcium oxide within quicklime reacts readily with water, liberating 267 kcal/kg CaO, as follows: CaO + H 2 O –> Ca(OH Developing a modular lime plant Cimprogetti
.jpg)
Lime/quicklime for metallurgy – how producing and briquetting
2023年2月4日 What is lime Lime, also known as quicklime or burnt lime, is mainly composed of calcium oxide, molecular formula CaO, which is a white block or powder cubic crystal The lime commonly used in industry will be dark gray due to impurities such as magnesium oxide, aluminum oxide and ferric oxide The relative density is 325338g/cm3, the true density is 2024年6月23日 Reactivity of QuickLime QuickLime, also known as calcium oxide, exhibits remarkable reactivity due to its chemical composition and properties Understanding the reactivity of QuickLime is crucial in various industrial processes and applications AcidBase Reactions: Quick Lime reacts vigorously with acids to form salts and waterQuickLime 101: Everything About This Super Substance ZME2017年7月7日 When limestone is used as a fluxing material then it is used as either raw limestone or as calcined limestone Hydrated lime is generally produced from high calcium quicklime and contains 72 % to 74 % calcium oxide with 23 % to 24 % chemically combined water Processing of limestoneLimestone – Its Processing and Application in Iron and2021年5月20日 Calcium oxide (and calcium hydroxide) is also an important chemical for raising the pH of potable water and wastewater during its treatment However, there are different methods used to utilize quicklime during the different stages of water softening, neutralization, and stabilization, so it is important to adhere to the types of lime recommended by ASTM C1529 Calcium Oxide: From Ancient Warfare to Modern Industry

Quicklime Manufacturer, Limestone, Calcium Oxide Supplier
1) We have selfowned quarry with a deposit of 2 billions tons of limestone enabling us the long term supplying ability 2) The average content of Calcium Oxide in limestone is more than 54% 3) The burning equipment are the most advanced rotary kilns in the world which make sure low unburnt rate, high activity of CaO ( 430ml)This leaves quicklime, or calcium oxide (CaO), a material that has been used by humans throughout history Adding water to the quicklime will result in an exothermic reaction and the production of hydrated lime, or calcium hydroxide (Ca(OH) 2), a fine dry white powder not dissimilar to baby powder Adding more water to quicklime will produce a HOW TO CHOOSE THE RIGHT LIME PRODUCT• ASTM C25, “Standard Test Methods for Chemical Analysis of Limestone, Quicklime, and Hydrated Lime” • ASTM C1271, Standard Test Method for XRay Spectrometric Analysis of Lime and CHEMICAL ANALYSIS OF LIMESTONE/ CALCIUM OXIDE MATERIALS Limestone, or calcium carbonate, can be processed into quicklime, calcium oxide; slaked lime, solid calcium hydroxide; and lime water, which is aqueous calcium hydroxide These materials and the processes that turn one into another are Lesson Video: Limestone Nagwa

Quicklime Formula, Uses, Definition Britannica
2024年11月8日 The most common form of glass, sodalime glass, is composed of about 70 percent silica (silicon dioxide), 15 percent soda (sodium oxide), and 9 percent quicklime, with much smaller amounts of various other compoundsThe soda serves as a flux to lower the temperature at which the silica melts, and the quicklime acts as a stabilizer for the silica2023年10月11日 Calcium oxide was labelled the earliest chemical utilized by humans since it is an ionic material that people have used since the Middle Ages CaO Chemistry Calcium oxide’s chemistry: Calcium oxide has one cation and one anion The calcium cation with a valency of +2 and the oxygen anion with a valency of 2 make up the molecules of calcium Calcium Oxide (CaO) : Definition, Properties Uses2018年6月11日 When limestone (calcium carbonate; CaCO 3) is heated, carbon dioxide (CO 2) is driven off, leaving calcium oxide behind The reaction was probably discovered very early in human history because limestone is a common, readily available material in the form of chalk and sea shells, and the amount of heat needed to produce the reaction can easily be produced in Calcium Oxide Encyclopedia2023年10月11日 CALCIUM OXIDE (LIME, QUICKLIME) CALCIUM OXIDE (LIME, QUICKLIME) View in own window NAS/CWTC 00782Name:CALCIUM OXIDE (LIME, QUICKLIME)CAS No:Formula Weight:560Chemical Formula:CaO Description: Hard, white or grayishwhite porous pebble or powder, odorless, and quick slaking Solubility in water: 1 g/840 ml at limestone, quicklime and slaked lime
.jpg)
HOW TO CALCULATE EFFICIENCY OF YOUR LIME BURNING
Iron Oxide, Fe 2O 3 033% Calcium Oxide, CaO 4550% Magnesium Oxide MgO 816% Sulphuric Anhydride, SO 3 039% LOI (at 1,000 C) 4240% A test on the quicklime produced the following result: Table 2: Chemical analysis obtained on limestone (%) Silicon oxide, SiO 2 234%, Aluminium Oxide, Al 2O 3 063, Iron Oxide, Fe 2O 3 035%, Calcium Oxide, CaO 26062020 Lime is produced through the calcination of limestone (calcium carbonate) in a lime kiln at temperatures at or above 2000 degrees Fahrenheit The product of calcination of high calcium limestone is "quicklime" or calcium oxide Quicklime in turn can be reacted with water to produce hydrated lime (calcium hydroxide) Read Morehow is quicklime produced Welcome to Tables Thyme2024年7月28日 No Quicklime or burnt lime is calcium oxide (CaO) Hydrated lime is calcium hydroxide (Ca(OH)2) which is created by combining quicklime with water Is agricultural lime the same as quicklime? No Ag lime or garden lime is usually ground limestone or calcium carbonate (CaCO3) It does not have the same properties as purified quicklimeWhere to Buy Quicklime A Complete Guide to Sourcing and 2021年12月20日 The Importance of Calcium Carbonate Calcium carbonate (CaCO3) comprises more than 4% of the earth’s crust and is found worldwide Its most common natural forms are chalk, limestone, and marble (produced by Calcium Carbonate Manufacturing Process and
.jpg)
High Calcium Quicklime Carmeuse
High calcium quicklime (CaO) is produced when limestone, or calcium carbonate (CaCO 3), is heated in a kiln through the process of calcination CaCo 3 + heat > Cao = CO 2 After limestone with high calcium content is sourced from our 2023年4月24日 This process, known as calcination, causes the limestone to decompose into calcium oxide and carbon dioxide Quicklime processing The calcium oxide produced in the lime kiln is then processed further to remove How is calcium oxide manufactured industrially?2023年10月11日 Quicklime (Calcium Oxide) quicklime is one of many reagents offered by Mintek Resources Quicklime, also referred to as lime (calcium oxide (CaO)), is derived from high quality, natural deposits of limestone (calcium carbonate (CaCO3)) or dolomitic limestone (calcium magnesium carbonate (CaCO3 + MgCO3) Quicklime is produced by heating the Quick Lime Preparation, Properties and Uses Hebei Yayang The conversion of limestone into quicklime (calcium oxide) and slaked lime (calcium hydroxide Chemguide: Core Chemistry 14 16 Just for the record, an igneous rock is one formed by the welling up of molten material from very deep in the earth limestone, quicklime and slaked lime chemguide

Quicklime Manufacturer, Limestone, Calcium Oxide Supplier
Quicklime Supplier, Limestone, Calcium Oxide Manufacturers/ Suppliers Shandong CITIC Calcium Industry Co, Ltd The average calcium oxide content is 54% min with stable quality we shall continue researching and developing new products in the deep processing field of limestone, such as food grade hydrated lime, stonepaper making, chemical reaction, so calcium hydroxide contains 757% CaO and 243% H 2O The process of adding water to calcium oxide to produce calcium hydroxide is referred to as hydration process or lime slaking The hydration of CaO, commercially referred to as quick lime, is an exothermic process releasing a great quantity of heatAn Overview of Lime Slaking and Factors That Affect the Process2023年2月10日 Quicklime, also known as calcium oxide (CaO), is a white or gray powder that is created by heating limestone to a high temperature, typically above 900°C (1652°F) This process, known as What Is Quicklime And How Does It Work? Medium2024年2月27日 Quicklime is produced through the thermal processing of limestone in industrial kilns During quarry operations, fine particulate quarry dust adheres to limestone lump surfaces, increasing the bulk concentration of impurities in limestone products During thermal processing in a kiln, impurities such as Si, Mg, Al, Fe, and Mn react with Ca, reducing quicklime product Impact of Limestone Surface Impurities on Quicklime Product

DETERMINATION OF TOTAL CALCIUM OXIDE IN LIME (IS: 1514
2015年5月4日 Ignite the residue in a platinum crucible, blast, cool in a desiccators and weigh as aluminium oxide and ferric oxide Make up the filtrate to 250 ml Pipette out 50 ml of the filtrate in a beaker and dilute to 100 ml Heat to boiling and add slowly about 35 ml of boiling ammonium oxalate solution